

 Assistant Professor David Kwabi, PhD Candidate Fawaz Ali, and PhD Candidate Fiki Owhoso, discuss setup of CO2 separation experiments.
Assistant Professor David Kwabi, PhD Candidate Fawaz Ali, and PhD Candidate Fiki Owhoso, discuss setup of CO2 separation experiments.
Climate change has presented a real crisis for our world, and there is a lot of research effort and policy development aimed at satisfying our energy needs by harnessing renewable power rather than burning fossil fuels. Nevertheless, the pace at which these new technologies are being adopted is slow, and capturing CO2 from the atmosphere is drawing attention as one of a few potential near-term strategies to mitigate the harmful effects of past and present fossil emissions on the climate.
David Kwabi, assistant professor of mechanical engineering, has received a 2021 NSF CAREER award for his proposal and work in carbon capture. His proposal entitled, "CAREER: Combining Electrode Engineering with Electrochemical Modeling to Enable Atmospheric CO2 Capture," aims to develop and study a new approach to energy-efficient CO2 separation from air.
If any carbon capture technology is to be practically viable and climate-friendly, it must, at the very least, be energy efficient and emit little CO2, if at all. Conventional CO2 capture processes are run with heat, which limits their energy efficiency; ironically, the heat required is often readily sourced by burning fossil fuels such as coal and natural gas. Powering CO2 capture with renewable electricity rather than heat is one way of addressing the efficiency and sustainability requirements.
"This research could address the problem by informing the design of electrochemical systems that run on renewable electricity while removing large amounts of CO2 from the atmosphere. These systems could be deployed at large scale, and the captured CO2 can be converted into commercially useful plastics, construction materials, agricultural products, and other chemicals."
The method being researched uses organic molecules which are attached to porous, electron-conducting electrodes to separate CO2 from air, all powered from renewable energy resources like wind power or solar power (see figure 1).
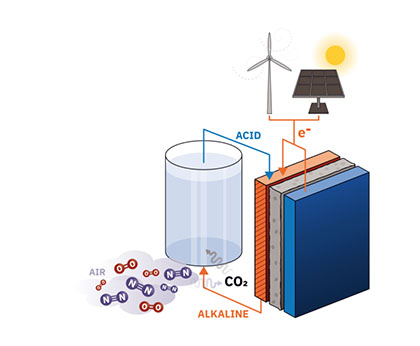 Figure showing the how renewable energy can power the separation of CO2 from air through a electrochemical flow battery cell.
Figure showing the how renewable energy can power the separation of CO2 from air through a electrochemical flow battery cell.
The organic electrodes will be integrated into an electrochemical flow cell, a type of battery specially designed to handle the flow of liquid during their operation. As the electrolyte is pumped through the electrode and a charging current is applied to the cell, protons are absorbed from the electrolyte and into the electrode, making the electrolyte basic and capable of selectively absorbing CO2 from any mixture of gases, including air. Reversing the direction of the charging current results in a discharging current that causes the absorbed protons to be released back into the electrolyte, making it acidic; this also releases pure CO2 gas, in much the same way a can of soda fizzes and releases CO2 when opened. In principle, several cycles of flow cell charging and discharging will allow the capture and concentration of a large cumulative amount of CO2.
"Our specific aim is to design the electrodes in such a way that they can store or release protons upon electric polarization, and thereby cause reversible changes in the acidity of a water-based electrolyte," explained Kwabi. The goal is for CO2 to be selectively absorbed as carbonate ions under basic conditions and then released as a more concentrated gas under acidic conditions.
By combining these experiments with characterization and modeling studies, Kawbi’s lab team will look into how the chemical composition of the electrode and the nature of its interface with the electrolyte, ultimately influence the rate and energy efficiency of the overall separation process.
The project has an educational plan to be executed in partnership with U-M’s Museum of Natural History, featuring informal discussion and hands-on scientific demonstrations centered around engaging Michigan residents on the needs for, benefits, and costs of CO2 capture and utilization technology.
"I'm excited about this project because it tackles an important societal problem, and lies at the intersection of electrochemical modeling, device design, and physical chemistry, all of which I'm passionate about," said Kwabi. "With the Global CO2 Initiative and several research groups at U-M making strides on various aspects of promoting carbon capture and utilization, it's a truly exciting time to be working in this area."
And as an added bonus, the captured CO2 may be converted into commercially useful plastics, construction materials, agricultural products, and other chemicals for a profit.