Mechanical Engineering Professor Jianping Fu’s paper entitled “Mechanosensitive subcellular rheostasis drives emergent single-cell mechanical homeostasis” is published by the journal of Nature Materials. Fu is also an Associate Professor of Biomedical Engineering and of Cell and Developmental Biology, as well as the Associate Director of the Michigan Center for Integrative Research in Critical Care.
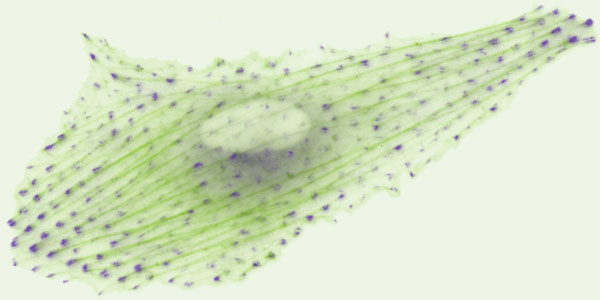
In this work, Dr. Fu and his co-workers have studied a well-known biomechanics phenomenon, termed “the cell mechanical homeostasis”. Cell mechanical homeostasis describes how mammalian cells can maintain their stable mechanical states under environmental perturbations, and has been a topic of intensive studies in the past mainly at the cellular level. It is known that dysregulation of cell mechanical homeostasis is involved in many pathophysiological conditions, such as cardiovascular diseases and cancer. In their research, Dr. Fu and his co-workers have obtained some interesting observations, suggesting that cell mechanical homeostasis is collectively driven by dynamic, graduated subcellular processes (“rheostasis”) involving some prominent subcellular structures including the actin cytoskeleton and focal adhesion.
For decades, the relationship between collective dynamics at smaller scales and the emergent system-level property at larger scales has attracted greatly scientists in biology, physics, and materials sciences. For living cells, which behave as autonomous adaptive systems operating through subcellular events, the link between subcellular dynamics and single-cell level properties has not been studied and understood. The work by Dr. Fu and his co-workers, for the first time, has established the missing link between an important single-cell property, namely the mechanical homeostasis, and collective subcellular behaviors. Intriguingly, they discover that subcellular dynamics would observe patterns different from that at the single-cell level, and thus have confirmed single-cell homeostasis as an emergent phenomenon. This observation has revealed a general and previously underappreciated physical property of single-cell behaviors, supporting multidimensional and interdisciplinary research to study cellular behaviors as emergent properties driven by collective dynamics at the subcellular level.
Understanding cell mechanical homeostasis from a subcellular angle would provide novel insights about their physical nature and regulation. Such new knowledge can potentially provide new avenues for treatments of diseases involving dysregulated cell mechanics.
Team members for this publication include Shinuo Weng (graduate student, Univ. of Michigan), Yue Shao (graduate student, Univ. of Michigan), and Weiqiang Chen (formal graduate student, now faculty at New York Univ.).
Read the paper: http://www.nature.com/nmat/journal/v15/n9/full/nmat4654.html
