Understanding how heat travels inside a material is critical to improving the performance, efficiency and reliability of the devices and systems in which it is incorporated. Thermoelectric refrigerators and power generators, heat sinks, power electronics, thermal barrier coatings and thermal interface materials all rely upon maximizing or minimizing heat transfer.
Light weight, low cost and corrosion resistance are some of the properties that make polymers ideal for use in the packaging of electronics such as smartphones or LEDs, or as structural components in cars or airplanes. However, while they have many appealing properties, they don’t conduct heat well, and this, in many cases, limits their use.
“Low thermal conductivity is a main factor limiting the expanded use of polymers in new applications,” said ME Professor Kevin Pipe. “Current plastics are thermal insulators. You can mix high thermal conductivity fillers such as metals or ceramics into a polymer to make it more conductive, but the increases in cost and weight often negate its original advantages.”
MOLECULAR DESIGN
In collaboration with Materials Science and Engineering Professor Jinsang Kim’s group, Pipe’s lab is using molecular design principles to improve thermal conductivity in polymers. While researchers have long studied how to engineer polymer molecules to have desired mechanical, electrical or optical properties (such as for use in organic LED displays), little work has focused on how to use similar principles to modify a polymer’s thermal properties.
As a result, the physical properties of polymers that determine their thermal conductivity are not well understood. “We’re still working to understand the fundamentals of how heat flows in polymer systems,” Pipe said.
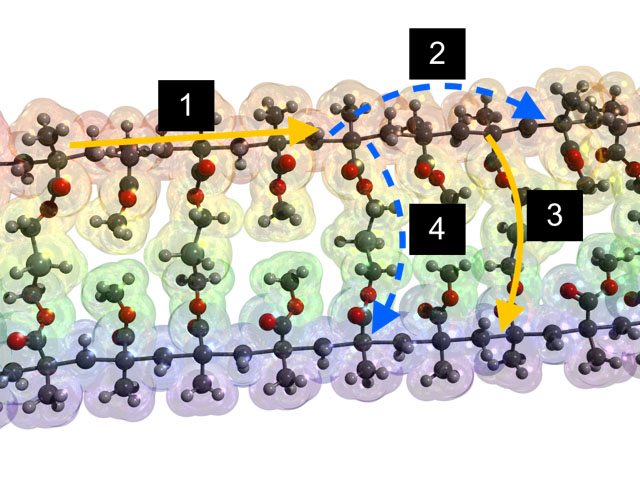
Most common polymers have a spaghetti-like molecular structure in which long polymer molecules are entangled in an amorphous rather than crystalline manner. It is generally believed that heat travels easily down the polymer backbones, but faces resistance when moving between backbones. In recent work, Pipe and Kim’s groups showed that carefully created links between the backbones using hydrogen bonds could allow heat to move through the structure more easily. Their work, published in Nature Materials in 2015, demonstrated a tenfold improvement in thermal conductivity using this method and achieved the highest thermal conductivity yet measured in an amorphous polymer.
Demonstrating this improvement in an amorphous polymer is important, since they are more amenable to scale-up than crystalline polymers, in which polymer chains are packed side by side in a regular pattern. While crystalline polymers are known to exhibit increases in thermal conductivity, these increases only occur in one direction (the direction of chain alignment), making them difficult to utilize in macroscale manufactured parts.
In another recent work, published in Science Advances in 2017, Pipe and Kim’s groups increased the pH of polyelectrolytes in solution, imparting electrical charge to the individual polymer segments that caused them to uncoil due to electric repulsion. This uncoiling, as well as related increases in backbone stiffness and packing that occurred when the molecules were used to create a solid film, increased thermal conductivity by a factor of six.
In collaboration with Materials Science and Engineering Professor John Kieffer’s lab, Pipe’s group is using computational methods such as molecular dynamics models to simulate the flow of heat between individual polymer chains.
Their recent work, published in Journal of Physical Chemistry B in 2017, resolved seemingly discrepant trends measured in the literature for the thermal conductivities of crosslinked polymers. The investigators showed that the role of crosslinkers in improving thermal transport is often not to act as conduits for heat conduction themselves but rather to bring the chains closer so that weaker but longer-range forces known as van der Waals interactions can transmit a larger amount of heat by involving a greater number of polymer segments (monomers).
ENHANCING THERMOELECTRIC ENERGY CONVERSION
Pipe’s group has also been studying thermoelectric effects in conducting polymers to improve the efficiency with which they can perform solid-state refrigeration or harvest waste heat to create electricity.
In particular, his group has examined the doping of polymers, which is used to control electrical carrier concentration and solubility. In work published in Nature Materials in 2013, Pipe’s group showed that excess non-conducting dopant molecules can inhibit the flow of electricity through the material; by carefully removing these excess dopants, they demonstrated the highest thermoelectric energy conversion efficiency yet achieved in a polymer or any organic semiconductor.
Pipe’s work has been funded as part of the Center for Solar and Thermal Energy Conversion, an Energy Frontier Research Center funded by the US Department of Energy, Office of Science, Basic Energy Sciences; the U-M Energy Institute and Samsung.
Photo caption (based on AR screenshot layout attached): LEFT: Heat transfer pathways along and between polymer chains by conduction through covalent bonds or longer-range van der Waals interactions. Image: J. Phys. Chem. B 121, 4600 (2017).
