If a harmful algal bloom has ever closed your favorite lake-side beach or caused your municipality to issue a drinking water warning, a possible cause is excess nitrogen, phosphorous and organic nutrients. These nutrients are present in the surface water runoff from farms, factories, and our own well-fertilized backyards, and the excess nutrient build-up has been identified as a major cause of eutrophication, or the explosive growth of algae in water bodies. This can pose environmental threats to aquatic species and result in health risks in human-beings (see figure 1). The increased production and utilization of nitrogen fertilizers combined with fossil-fuel combustion and contamination of groundwater and other surface-water bodies with reactive nitrogen-species, including nitrates (NO3-), nitrites (NO2-) and ammonia (NH3), has become an increasingly concerning issue.
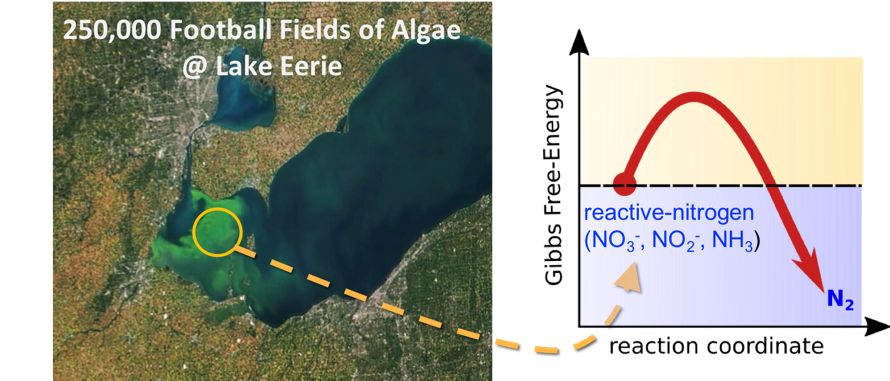
One area of particular concern to ME Assistant Professor Rohini Bala Chandran is the excess nitrogen-based nutrient buildup in wastewater. “Municipal wastewater treatment is energy-intensive requiring between 15 and 45 megajoules per kilogram of nitrogen-species present. Moreover, the overall energy consumption by public drinking water and wastewater utilities can often represent up to 40% of a municipality’s electricity use,” she said. “Water and wastewater treatment accounts for a surprising amount of the power consumption in the United States.”
Bala Chandran, who directs the U-M Transport and Reaction Engineering for sustainable Energy (TREE) Laboratory, also notes a bit of irony. “Some of the nutrients and chemical species present in wastewater could actually provide energy, yet we’re spending money and energy to get rid of them. Wastewater is a misplaced resource in that respect.”
State-of-the-art techniques to remediate nitrogen-based contaminants from wastewater are biological (microbial) and electrochemical ion-exchange processes. Both processes are typically energy intensive. Biological approaches may not be applicable for all waste streams, especially industrial effluents that harbor conditions unsuitable for microbial growth, and electrochemical ion-exchange approaches commonly applied at the industrial scale for drinking water applications often result in the generation of secondary concentrated waste streams.
Bala Chandran’s research group, including ME doctoral student Luisa Barrera and ME senior Erika Brower, is developing more sustainable and scalable options. More specifically, her team is developing processes and devices that use renewable energy sources to pair energy and nutrient recovery from wastewater contaminants.
“Our goal is to identify solar-powered wastewater nitrate treatment pathways that facilitate the recovery of energy by producing value-added chemicals from these nutrients,” said Bala Chandran. The work is funded by Bala Chandran’s startup funds and MCubed, a University initiative to encourage innovative, interdisciplinary research. The project marries fundamental materials-scale catalysis, physics-based modeling, and experimental investigations.
Energy recovery
Fundamental efforts include gaining physical insights into understanding different nitrogen reduction pathways that yield value-added products, such as: ammonia (NH3) for use directly as a fuel or ammonium (NH4+) in dissolved form that can be used in fertilizer production and nitrous oxide (N2O) for use as an oxidizer in the burning and combusting of fuels, including in turbocharged automobile and jet engines.
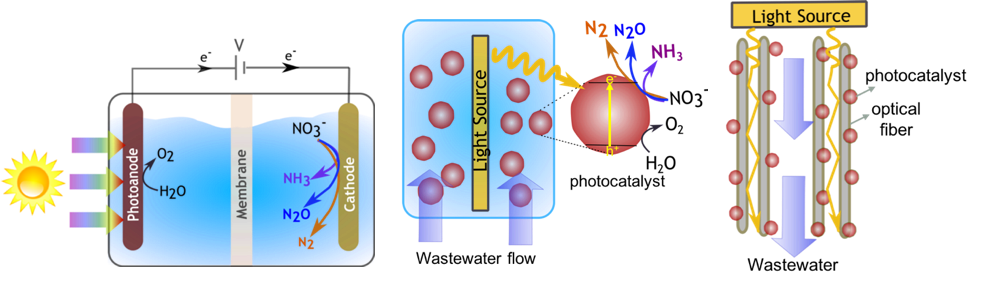
An experimental component entails reactor prototyping, design, and demonstration of a solar-powered photocatalytic reactor to recover the energy and nutrients from the wastewater nitrates.
“Since we don’t yet fully understand which nitrate reduction pathway is the most effective in maximizing energy recovery from the treatment process and which materials and device-scale designs help attain the most optimal process efficiencies, we’re developing predictive physics-based models to determine the energy and nutrient recovery potential of the different reduction pathways and to guide materials selection and overall device design,” Bala Chandran explained.
The reactor design concepts Bala Chandran is developing will achieve a greater reactive surface area per unit volume and enhanced light and mass transport and therefore result in a more efficient utilization of materials and incident sunlight. Her group plans to accomplish this by exploring fluidized-bed photocatalytic particle reactors and fixed-bed photocatalysts immobilized on optical fibers (see figure 2).
“As we improve species transport by maximizing reactive surface area, we improve the overall efficiency of our system,” she said. The concepts under development also enable scalability – a challenge for photocatalytic reactor designs that have commonly used planar electrode architectures.
Preliminary results have identified efficiency limits for the different nitrate reduction pathways (see figure 3) as a function of an important material properties– the photocatalyst bandgap and the electrocatalytic properties.
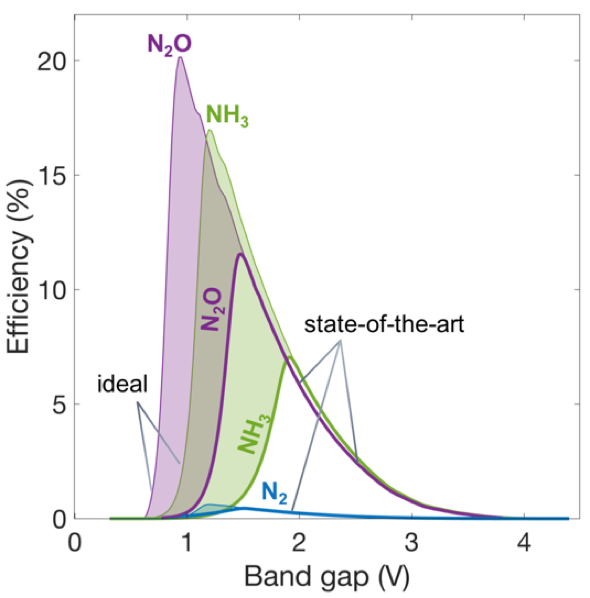
“Our results show we can attain solar energy conversion efficiencies of up to 11% and 7% respectively for producing nitrous oxide and ammonia with the state-of-the-art catalysts,” noted Bala Chandran.
The results also indicate there is scope for improved efficiencies aided by new materials discovery and design, because the group observed a two-fold increase in the efficiency values for all the products formed (figure 3) when ideal electrocatalysis for the nitrate reduction reaction was assumed.
Solving grand challenges
Bala Chandran’s work is paving the way toward new, more sustainable and scalable ways to approach wastewater treatment. This is critical to help balance the global nitrogen cycle, a problem the National Academy of Engineering has designated a grand challenge for engineering in the 21st century.
As with all grand challenges, the work transcends disciplines. It requires not only mechanical engineering expertise but also knowledge of materials science, chemical engineering, and chemistry.
Next steps include developing an experimentally validated modeling framework for understanding the combined influences of various physical phenomena and prototyping and evaluating reactor components against those of state-of-the-art reactors to measure performance and predict efficiencies.
“From there, we’ll work on the design and fabrication of the reactor itself,” noted Bala Chandran, “with a focus on first prototyping the improvements we think will make a real difference.”
Making a difference is crucial, she added. “We need to find new processes that don’t just destroy but also recover.”
