Endoscopic microscopes are very small instruments that can enter the human body, often through the GI tract or the esophagus, and capture imagery. Physicians and scientists can then study that imagery to understand the conditions and behaviors of cells in vivo, or in a living organism. In clinical settings, these devices are often employed to search for cancers or other diseases and, in research settings, to study cellular behavior under natural circumstances.
The microscope on the end of most of these devices, once inside the human body, is able to examine the surface of living organ tissue through the use of white light imagining. “White light imaging is like when you take a picture with a camera. You get your image of the light you’ve collected more or less as you would see with your eyes,” said Kenn Oldham, professor of mechanical engineering.
Oldham and his lab have received a $1.7 million R01 grant from the National Institute of Health for their project, “3D fluorescence imaging with high-speed, addressable laser scanning in freely-behaving mice.” The aim for this project is to develop a new endoscopic microscope capable of taking images beneath the surface of organ tissue and at cellular resolutions. Particularly, the lab team aims to tailor the device for neuron imaging.
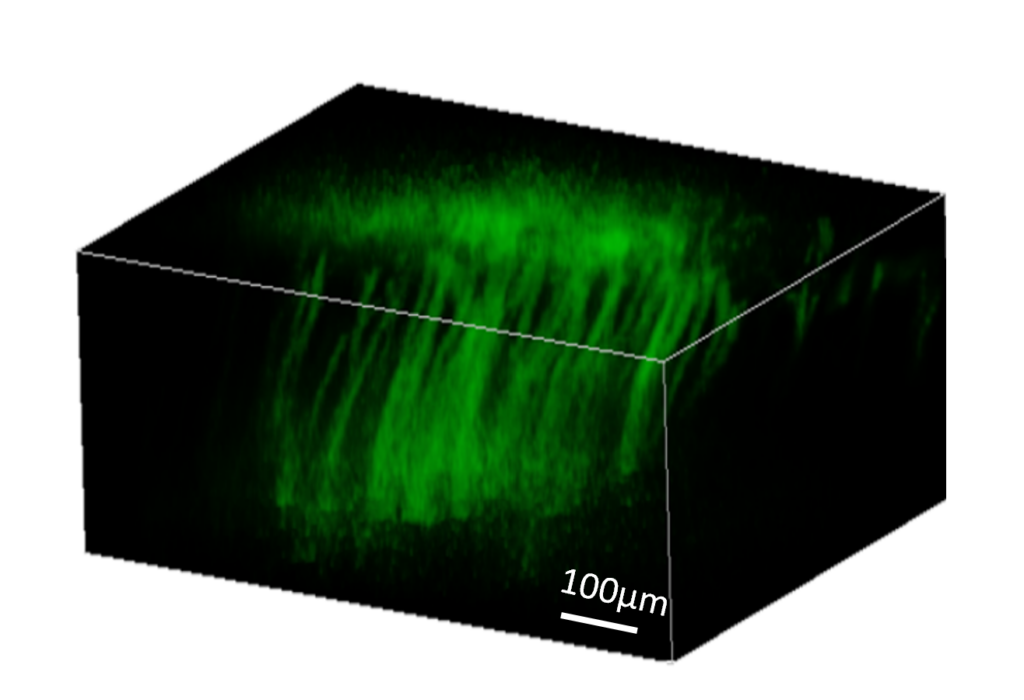
“We’re looking at behaviors in the upper few hundred microns of tissue,” Oldham said. “But it’s still a big deal because that gives you an image of cellular behavior vertically through tissue, and that’s something that scientists will often look at to understand behavior. Just looking at a single plane or the surface doesn’t give you all that information.”
Normally, researchers and physicians have to take biopsies, or perform their studies post-mortem, to gain an understanding of cellular behavior beneath the surface of organ tissue. These methods, however, do not offer the same depth of analysis, because the tissue will no longer be under natural circumstances.
The Oldham group has been able to clear this hurdle by making use of multiphoton microscopy. “This process uses a very short intense pulse of high intensity, but long wavelength, laser light to penetrate relatively deep into tissue,” Oldham said. “Relatively deep to us is like half a millimeter.”
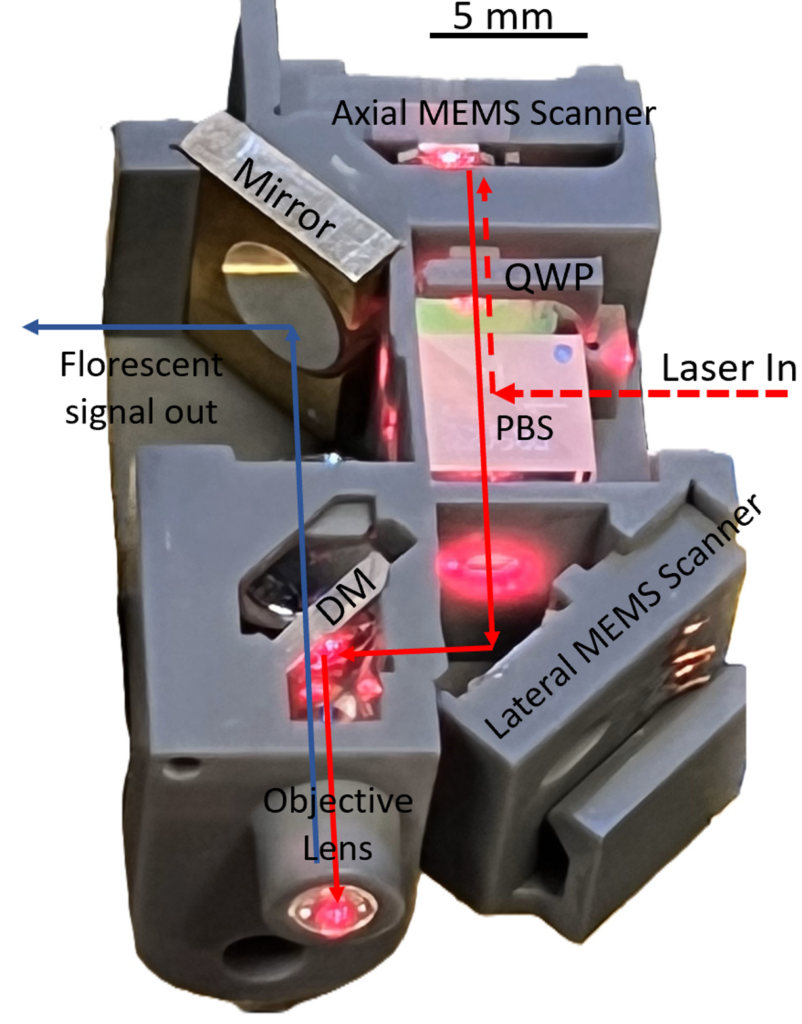
The device works by converting electrical signals into motion to manipulate very small mirrors, just a few millimeters in diameter. This allows researchers to direct laser light through organ tissue and then deliver that light back to a receiver.
“Something unique to the University of Michigan is that our mirrors can move vertically and at high speed change the focus depth of the instrument into tissue,” said Oldham. “And that allows you to even go beyond just taking a plane of cellular images.” Researchers can, in fact, use the device to take a series of images at different planes that, when stacked together, show cellular behavior within a three-dimensional cube of tissue.
“We also have the ability to manipulate our scanning patterns – which is how we direct the light through the tissue. If you have to scan all of the area, all of the time, you can’t take images particularly quickly, but our actuators have some control ability that allows us to pinpoint specific locations and then scan that narrow region more quickly,” said Oldham. “This allows us to get sample data faster than most of the other technologies in this space, if we’re successful.”
The hope for these microscopes is that they will be able to take imagining twice as deep as existing technologies currently allow and to do so faster, which will hopefully allow for earlier detections of cancers and aid the development of treatments for degenerative neurological diseases, like Alzheimer’s.
“There’s a story behind this NIH award—a tale of dreams, determination, and discovery. What started as a curious inquiry evolved into a groundbreaking project in brain imaging, driven by a passion for understanding the unknown. Our innovative leap in miniaturizing complex imaging systems into a compact ensemble of light, lenses, and mirrors has been a journey of pure exhilaration, marking a significant breakthrough in our field,” said Taybeh Sahraeibelverdi, a recently conferred graduate of ME’s PhD program and the researcher who developed and prototyped many of the early stage instruments for this project. “This award is not just a recognition of our relentless pursuit but a validation of our vision for transforming neuroscience. It underscores our commitment to scientific advancement and resilience. Last but not least, I want to express my gratitude to Joonyoung Yu, Dr. Ahmad Shiraz, and Miki Lee for their invaluable support in this project.”
