Activity trackers, smart watches, glucose monitors, and other wearable biosensor technology are a growing part of our lives. Now, ME Research Associate Professor Mihaela Banu is putting her research where our mouths are, with a collaborative initiative to develop a new smart, cyber-physical dental implant system.
The University’s Mcubed program, designed to spur innovative, multidisciplinary solutions to current problems, has brought together a cohesive and productive team of mechanical engineers and dentists. Their collaboration contributes to the 2030 roadmap of the National Institute of Dental and Craniofacial Research and a project campaign to accelerate development of oral biodevices by advancing technologies.
The system Banu and colleagues are developing overcomes several challenges of current artificial implants and is likely to enhance patient outcomes, overall health and quality of life.
“Intelligent dental implants have the real potential to improve patients’ lives,” said Laurie McCauley, dean of the U-M School of Dentistry and a collaborator with Banu’s team, along with Gustavo Mendoca, DDS, MS, PhD, clinical associate professor, and Sun-Yung Bak, DDS, clinical assistant professor.
“Many people, particularly those with health problems that affect their bones or who have had cancer treatment — and healthy people, too — are suffering with issues related to tooth loss,” Banu added, “and we probably can help them.”
Worldwide, nearly 160 million people suffer from edentulism, or toothlessness. In the United States, about 15 million people have crown and bridge replacements for natural teeth, and over three million have implants. The use of implants will only rise as the population ages and the incidence of health conditions and treatments that lead to bone loss, such as diabetes, osteoporosis, periodontitis, and cancer therapy, also increases.
Implants in healthy patients with good bone integrity boast high early-success rates but over time, the risk of failure grows, in many cases caused by an inflammatory reaction known as periimplantitis. This can lead to serious infection and the need for implant removal. In less healthy patients, unfortunately, the risks are greater and the time until possible failure is shorter.
Improving compatibility through porosity
For success over the long term, implants must be stiff enough to withstand the stresses caused by chewing, which leads to perpendicular loading as well as lateral and rotational displacements. Together, these create nonuniform stresses on the bone that, over time, can lead to trouble.
In finite element simulations, Banu’s group has shown that even very small displacements or rotations of the implant will generate nonuniform stress points, which lead to inflammation. And this process, when it occurs repeatedly, often results in infection. A mismatch between the stiffness of the implant and stiffness of the bone also contributes to nonuniform stresses.
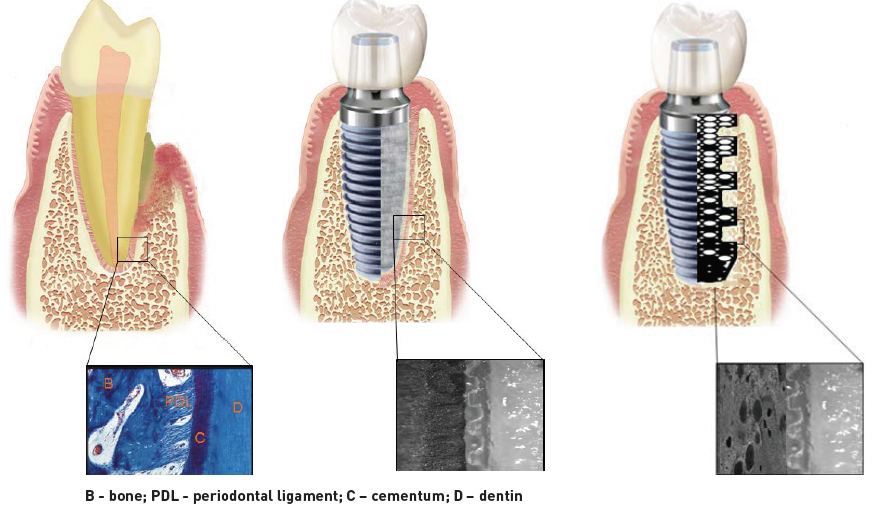
Today’s implants, primarily made from titanium and its alloys, are solid, with the surface treated through chemical etching or laser ablation to create pores. The roughened surfaces facilitate bone cell adhesion and growth around the wall of the implant, a process known as osteointegration. Using a new approach, Banu and her doctoral student Jaekwang Shin have introduced porosity inside the implant and demonstrated that doing so gives bone cells greater, and deeper, surface area for adhesion.
“The specific porosity we introduced has two important effects,” Banu said. “It offers greater stability and it decreases the stiffness of the implant. In this way, we can match the stiffness of the implant to the bone more closely and mitigate much of the nonuniform stresses.”
Novel manufacturing approaches
While conventional implants are produced by cutting and milling a metal rod, Banu’s group has demonstrated another concept. She and her team are using 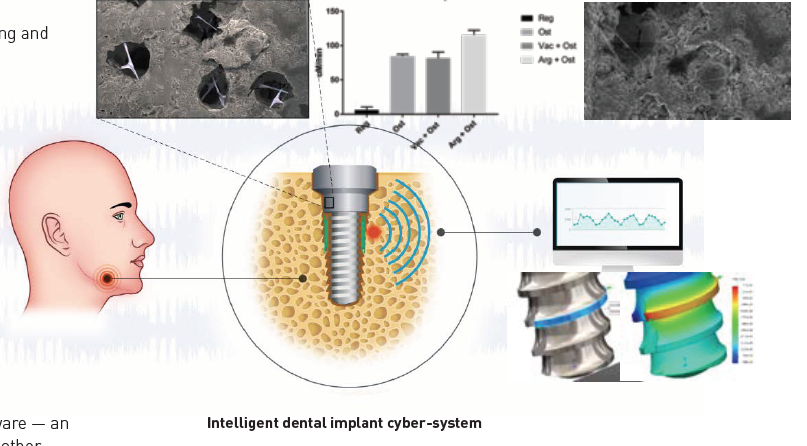 selective laser sintering of titanium (and the alloy Ti6Al4V) powder and resin to create a porous titanium foam. Future work will also use another additive 3D manufacturing process, direct metal printing.
selective laser sintering of titanium (and the alloy Ti6Al4V) powder and resin to create a porous titanium foam. Future work will also use another additive 3D manufacturing process, direct metal printing.
During the manufacturing process, a sensor will be embedded in the implant to monitor displacement and stresses. This solution was developed together with Bogdan I. Popa, assistant professor in ME. The intriguing design and its manufacturability attracted Robert Buechler, a senior ME student, to be deeply involved in realizing the system’s first virtual version.
The sensor will be interrogated wirelessly and passively and therefore won’t require a power source. Signals from the sensor will be recorded by software — an app installed on the user’s smartphone, watch, tablet, or other mobile devices. The data can then be used to predict the evolution of implant mobility and to detect possible failures early. This will enable patients to seek treatment before symptoms develop.
To date, Banu’s team has manufactured a prototype system and, in vitro, demonstrated an improved osteointegration rate compared to a solid titanium structure. Postdoctoral fellow Jessica Alfonso Ferreira, DDS, was integral to obtaining these results.
The team has been working together since 2015. “It’s been a close and productive collaboration,” Banu said. “Few other places in the world have this caliber, depth, and breadth of engineering and biomechanical expertise.”
